Abstract
Background: Salvage chemotherapy followed by high-dose therapy (HDT) and autologous stem-cell transplantation (ASCT) is an option to treat young patients (pts) with relapsed/refractory (R/R) diffuse large B-cell lymphoma (DLBCL). A complete remission before ASCT is the most important prognosis factor for a better outcome. Selinexor is a first-in-class, oral selective inhibitor of nuclear export which, through XPO1 blockade, causes nuclear accumulation and activation of tumor suppressor proteins, reduction in oncoproteins and cancer cell apoptosis. Selinexor has been approved by the US Food and Drug Administration for the treatment of R/R DLBCL, including de novo or transformed from follicular lymphoma (FL) pts after ≥2 therapies.
The phase Ib SELINDA (EUDRACT 2015-005612-15) study assessed safety and efficacy of selinexor, in combination with R-GDP for pts with R/R B-cell lymphoma.
Patients & methods: Eligible pts <70 years with R/R B-cell lymphoma after first or second treatment failure received every 21 days (d) 3 cycles of rituximab 375 mg/m² on D1, dexamethasone 40 mg on D1 to 4, cisplatin 75 mg/m² D1 and gemcitabine 1000 mg/m² on D1 and 8 (R-GDP) in combination with escalating doses of selinexor. Two dose levels of selinexor, 40mg and 60mg, were evaluated via 3+3 design. Initial dosing of selinexor for dose level 1 (DL1) was 40 mg on D1, 3, 8, 10. The dosing was modified to 40mg and 60mg on D1, 8, 15 for DL1 and DL2, respectively. The primary endpoint of SELINDA was the determination of the recommended phase 2 dose (RP2D) of selinexor in combination with R-GDP. Secondary and exploratory endpoints were safety, efficacy, and feasibility of ASCT after selinexor-R-GDP.
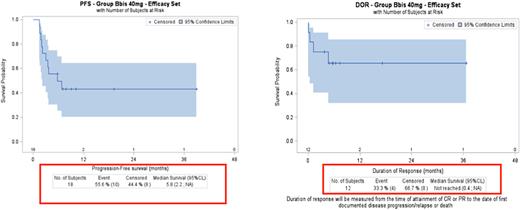
Results: The R2PD for selinexor in combination with R-GDP was established as 40mg on days 1, 8, and 15 (Maerevoet, IMCL 2021#176). Between January 2017 and January 2021, 32 pts received selinexor-R-GDP. We focused on the 18 pts who received the R2PD: 15 had DLBCL, 2 FL, 1 marginal zone lymphoma. In this cohort, median age was 61 years (range 44-69); 14 pts (78%) had stage III/IV, 6 had disease refractory to last treatment, 12 had relapsed. Thirteen pts received 1 previous line of treatment before inclusion, 5 pts received 2 previous lines. Four pts prematurely discontinued treatment: 2 for thrombocytopenia, 1 for COVID, 1 for disease progression. Major adverse events (AEs) in >10% of pts were reversible neutropenia (55 %), thrombocytopenia (44 %), and nausea (22%). No fatal AEs were observed. One patient is not evaluated, 7 pts (39%) achieved a complete metabolic response (CMR), 5 pts (28%) partial metabolic response (PMR) and 5 pts (28%) stable disease or progressive disease. According to Lugano criteria's, overall response rate (ORR) assessed at the end of treatment was 67%. Nine of the 15 pts (60 %) with DLBCL had metabolic response (CMR: 4, PMR: 5). Per protocol, peripheral stem cell collection and ASCT were optional, 4 pts of this RP2D cohort proceeded to high dose therapy with BEAM conditioning and ASCT. For this RP2D cohort, the 1-year overall survival is 72.2% and the 1 Year-PFS is 45%. The median duration of response was not reached (95%CI: 0.4-NA) (figure 1)
Conclusion: This study established the safety profile of weekly 40mg of selinexor in combination with R-GDP for R/R B cell lymphoma with an ORR of 67%, the median DOR is not reached. Reversible AEs were as expected for a platinum combination regimen. An ongoing randomized phase 2 study comparing R-GDP with or without selinexor in pts with R/R DLBCL will further evaluate the safety and efficacy of the combination.
Disclosures
Cartron:MabQi, Ownards Therapeutics, Abbvie, Roche, Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees; Gilead, Novartis, Mylteni, Sanofi, Abbvie, Takeda, Roche, Janssen, Celgene, Novartis, Bristol Myers Squibb: Honoraria. Morschhauser:Roche: Consultancy, Membership on an entity's Board of Directors or advisory committees; Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees; Genmab: Membership on an entity's Board of Directors or advisory committees; Miltenyi: Membership on an entity's Board of Directors or advisory committees; Allogene therapeutics: Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees. Thieblemont:Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Incyte: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bayer: Honoraria; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodations, expenses; Kite: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodations, expenses; Gilead Sciences: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodations, expenses; Cellectis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodations, expenses; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodations, expenses; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodations, expenses; Roche: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodations, expenses, Research Funding; Hospira: Research Funding; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodations, expenses, Research Funding; Bristol Myers Squibb: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodations, expenses, Research Funding. Bouabdallah:Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Kite/Gilead: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Sandoz: Consultancy, Honoraria; Abbvie: Honoraria; Incyte: Honoraria, Membership on an entity's Board of Directors or advisory committees; Milteny Biomedicine: Honoraria, Membership on an entity's Board of Directors or advisory committees. Feugier:AstraZeneca, Janssen, Abbvie, Beigene, Gilead: Membership on an entity's Board of Directors or advisory committees, Other: Congress Invitations. Tilly:Incyte: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees, Research Funding; ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal